A) 130 °C
B) 290 °C
C) 480 °C
D) 560 °C
E) 720 °C
G) D) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Two pure samples of atoms, labeled A and B, contain titanium (Ti) atoms and carbon (C) atoms, respectively.Each sample contains the same number of atoms.What is the ratio of the mass of sample B to that of sample A, mB/mA? Note the following atomic masses: C = 12 u; Ti = 48 u.
A) 1.0
B) 0.25
C) 2.0
D) 0.50
E) 4.0
G) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Complete the following statement: The internal energy of an ideal monatomic gas is
A) proportional to the pressure and inversely proportional to the volume of the gas.
B) independent of the number of moles of the gas.
C) proportional to the Kelvin temperature of the gas.
D) dependent on both the pressure and the temperature of the gas.
E) a constant that is independent of pressure, volume or temperature.
G) B) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The density of diamond, a form of carbon, is 3520 kg/m3.If the atomic mass of carbon is 12.011 u, how many carbon atoms are there in a solid diamond sphere with a radius of 0.0125 m?
A) 1.15 × 1027 atoms
B) 5.76 × 1026 atoms
C) 2.88 × 1025 atoms
D) 1.44 × 1024 atoms
E) 7.21 × 1023 atoms
G) B) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Calculate the rms speed of the carbon dioxide molecules in the air if the temperature is 15.0 °C.Note: The mass of the carbon dioxide molecule is 44.01 u.
A) 316 m/s
B) 469 m/s
C) 378 m/s
D) 404 m/s
E) 511 m/s
G) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A bubble with a volume of 1.0 cm3 forms at the bottom of a lake that is 20.0 m deep.The temperature at the bottom of the lake is 10.0 °C.The bubble rises to the surface where the temperature is 25.0 °C.Assume that the bubble is small enough that its temperature always matches that of its surroundings.What is the volume of the bubble just before it breaks the surface of the water?
A) 2.1 cm3
B) 2.8 cm3
C) 3.1 cm3
D) 6.0 cm3
E) 7.7 cm3
G) B) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A sample of neon gas at 20 °C is confined to a cylinder with a movable piston.The gas is then heated until its pressure is doubled.What is the final temperature of the gas?
A) 10 °C
B) 40 °C
C) 313 °C
D) 586 °C
E) This cannot be found since the final and initial volumes are unknown.
G) D) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Complete the following statement: The pressure exerted by a monatomic, ideal gas on the walls of its containing vessel is a measure of
A) the molecular kinetic energy per unit volume.
B) the average random kinetic energy per molecule.
C) the temperature of the gas, regardless of the volume of the vessel.
D) the total internal energy of the gas, regardless of the volume of the vessel.
E) the momentum per unit volume.
G) B) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Note the following six properties: 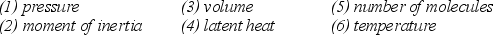 Which four of the listed properties are needed to describe an ideal gas?
Which four of the listed properties are needed to describe an ideal gas?
A) 1, 2, 4, 6
B) 1, 3, 5, 6
C) 1, 3, 4, 6
D) 1, 4, 5, 6
E) 2, 4, 5, 6
G) B) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Two moles of a monatomic gas with an rms speed of 294 m/s are contained in a tank that has a volume of 0.12 m3.If each gas particle has a mass of 8.220 × 10-26 kg, what is the absolute pressure of the gas?
A) 2.1 × 105 Pa
B) 1.1 × 104 Pa
C) 4.8 × 104 Pa
D) 2.4 × 104 Pa
E) 3.0 × 104 Pa
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A canister containing 115 kg of an ideal gas has a volume of 6.5 m3.If the gas exerts a pressure of 4.0 × 105 Pa, what is the rms speed of the molecules?
A) 260 m/s
B) 180 m/s
C) 310 m/s
D) 390 m/s
E) 420 m/s
G) B) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The volume of a carbon dioxide bubble rising in a glass of beer is observed to nearly double as the bubble rises from the bottom to the top of the glass.Why, according to our textbook, does the volume nearly double?
A) The temperature at the bottom is cooler than it is at the top.
B) The amount of carbon dioxide in the bubble increases.
C) The fluid pressure of the beer is greater at the bottom of the glass than at the top.
D) The pressure inside the bubble decreases as it rises.
E) The shape of the glass determines the net force exerted on the bubble.
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many moles are in a 0.53-kg sample of sulphur dioxide, SO2? (atomic masses: C = 32 u; O = 16 u)
A) 5.2
B) 8.3
C) 48
D) 1.6 × 104
E) 5.0 × 1024
G) All of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
An automobile tire is inflated to a gauge pressure of 32 lb/in2 at a temperature of -10.0 °C.Under strenuous driving, the tire heats up to 40.0 °C.What is the new gauge pressure if the volume of the tire remains essentially the same? (atmospheric pressure = 14.7 lb/in2)
A) 17.5 lb/in2
B) 20.6 lb/in2
C) 38.0 lb/in2
D) 40.9 lb/in2
E) 55.6 lb/in2
G) D) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A sealed container has a volume of 0.020 m3 and contains 15.0 g of molecular nitrogen (N2) , which has a molecular mass of 28.0 u.The gas is at 525 K.What is the absolute pressure of the nitrogen gas?
A) 3.9 × 10-19 Pa
B) 4.3 × 10-5 Pa
C) 1.2 × 105 Pa
D) 1.9 × 105 Pa
E) 4.3 × 106 Pa
G) C) and D)
Correct Answer

verified
Correct Answer
verified
Showing 41 - 55 of 55
Related Exams